On November 11, Nature published online the latest findings of Prof. Bao-Liang Song’s team from College of Life Sciences, Wuhan University. The research reveals the mechanism of cholesterol biosynthesis induced by feeding. This research will help to understand the regulation of cholesterol metabolism in the human body and lay the foundation for the treatment of metabolic diseases such as hyperlipidemia, obesity, fatty liver and diabetes.
The paper is entitled “Feeding induces cholesterol biosynthesis via the mTORC1-USP20-HMGCR axis”. The co-first authors are Dr. Xiao-Yi Lu, A.P. Xiong-Jie Shi, Dr. Ao Hu and Dr. Ju-Qiong Wang. Dr. Yi Ding, Dr. Wei Jiang, Dr. Ming Sun, A.P. Xiaolu Zhao, A.P. Jie Luo and Prof. Wei Qi are co-authors. The corresponding author is Prof. Bao-Liang Song.
Cholesterol is an essential lipid and its synthesis is nutritionally and energetically costly. High levels of cholesterol and other lipids are major risk factors for cardiovascular disease, nonalcoholic fatty liver disease, obesity and diabetes. Animals have adapted to an inconsistent food supply and cycles of famine (fasting) and feasting (feeding). It is well established that cholesterol biosynthesis is repressed in the fasting state and increases upon feeding. However, the mechanism is not well defined.
The study showed that the deubiquitylase ubiquitin-specific peptidase 20 (USP20) stabilizes HMG-CoA reductase (HMGCR), the rate-limiting enzyme in the cholesterol biosynthetic pathway, in the feeding state. The post-prandial increase in insulin and glucose concentration stimulates mTORC1 to phosphorylate USP20 at S132 and S134; USP20 is recruited to the HMGCR complex and antagonizes its degradation. The feeding-induced stabilization of HMGCR is abolished in mice with liver-specific Usp20 deletion and in USP20(S132A/S134A) knock-in mice. Genetic deletion or pharmacological inhibition of USP20 markedly decreases diet-induced body weight gain, reduces lipid levels in the serum and liver, improves insulin sensitivity and increases energy expenditure. These metabolic changes are reversed by expression of the constitutively stable HMGCR(K248R). This study revealed an unexpected regulatory axis from mTORC1 to HMGCR via USP20 phosphorylation and suggests that inhibitors of USP20 could be used to lower cholesterol levels to treat metabolic diseases including hyperlipidaemia, liver steatosis, obesity and diabetes.
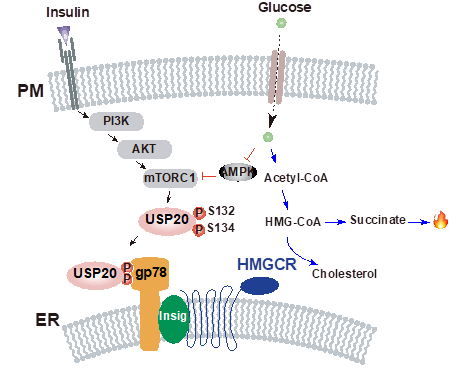
Figure: A simplified model depicting the regulatory mechanism of USP20 by insulin and glucose signalling pathways. The active mTORC1 phosphorylates USP20 at S132 and S134, which promotes the interaction with gp78, stabilizing HMGCR. Inhibition of USP20 renders the generation of succinate that further induces thermogenesis. ER, endoplasmic reticulum; PM, plasma membrane.
Article link: https://www.nature.com/articles/s41586-020-2928-y

