On June 8th, 2020, Professor Liang's Lab from the College of Life Sciences at the Wuhan University published a research article titled “Cryo-EM structure of an amyloid fibril formed by full-length human prion protein" on Nature Structural & Molecular Biology. This article reports cryo-EM structure of an amyloid fibril formed by full-length human prion protein. The first author of this article is Li-Qiang Wang, a young PhD student at Liang’s Lab, and Kun Zhao also contributed equally. Prof. Yi Liang and Prof. Cong Liu are the corresponding authors and jointly supervised this work. This work was supported by the National Natural Science Foundation of China and the Major State Basic Research Development Program.
Prions, meaning ‘protein infectious agents’, were initially described by S. B. Prusiner. Prion diseases are caused by the misfolding of prion protein (PrP). Misfolded PrP forms protease-resistant aggregates in vivo (PrPSc) that are able to template the conversion of the native form of the protein (PrPC), a property shared by in vitro-produced PrP fibrils. In this article, Yi Liang and his colleagues produced amyloid fibrils in vitro from recombinant, full-length human PrPC (residues 23–231) and determined their structure using cryo-EM, building a model for the fibril core comprising residues 170-229. The PrP fibril consists of two protofibrils intertwined into a left-handed helix. Lys194 and Glu196 from opposing subunits form salt bridges, creating a hydrophilic cavity at the interface of the two protofibrils. By comparison with the structure of PrPC, Yi Liang and his coworkers propose that two a-helices in the C-terminal domain of PrPC convert into six b-strands stabilized by a disulfide bond in the PrP fibril. These data suggest that different PrP mutations may play distinct roles in modulating the conformational conversion. Therefore, a cryo-EM structure of amyloid fibrils formed in vitro with recombinant human PrP provides insights into fibril architecture and the potential role of disease mutations.
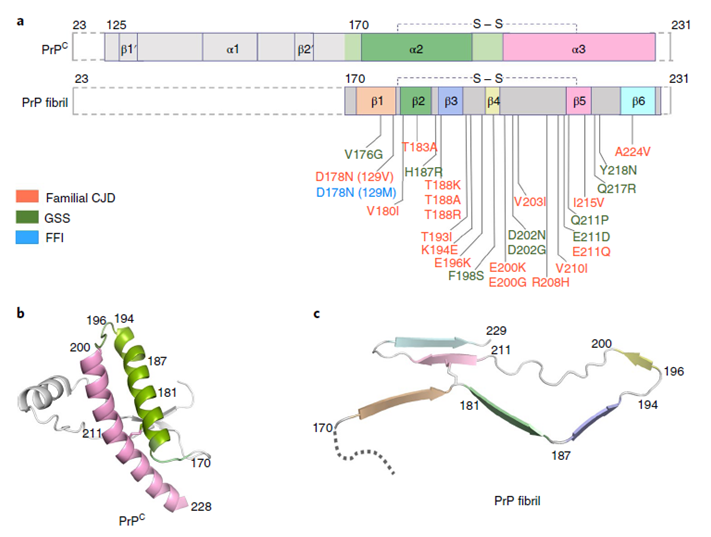
Comparison of the structures of PrPC and PrP fibrils
Link website: https://www.nature.com/articles/s41594-020-0441-5

